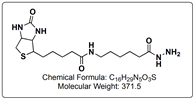
There are numerous biochemical reagents that utilize the NHS ester functional group. This efficient N-Hydroxysuccinimide reactive group was first introduced in 1975 by Bragg and Hou1. NHS esters, with a few exceptions, tend to be insoluble in aqueous buffers, which generally require them to first be dissolved in an anhydrous organic solvent, such as DMSO or DMF, prior to being added to the buffered aqueous solution containing the protein molecule of interest. 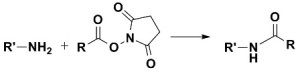 Another variety of these reagents, the N-Hydroxysulfosuccinimide group (Sulfo-NHS), does possess a water soluble property, allowing these reagents to be dissolved directly in aqueous buffer. However, many users still dissolve Sulfo-NHS containing crosslinkers and protein modifiers in organic solvents prior to use. The reason for this is twofold. First, when dissolved in organic solvent, the propensity for the reagent in the stock solution to hydrolyze is much lower than when dissolved in water. Secondly, the solubility of Sulfo-NHS containing reagents is considerably higher in DMSO or DMF than in aqueous buffers. For example, in DMSO, many reagents with the Sulfo-NHS ester functional group can be dissolved at concentrations as high as 50 mg/mL, whereas concentrations of 5 to 10 mg/mL are more typical for Sulfo-NHS esters in water. It is important to note that in order to maintain NHS and Sulfo-NHS activities in organic solvent, the solvents should have very low water content, such as CovaChem’s Molecular Biology Grade DMSO (CovaChem 18252) or DMF (CovaChem 18251). Many of these amine containing sample reactions with Sulfo-NHS and NHS esters are performed directly in an organic solvent, which often simplifies the procedure and gives the scientists more product isolation options. When carried out in dry solvent, these reactions tend to eliminate the hydrolysis by-products, and often result in the higher modification efficiencies, and require less NHS or Sulfo-NHS ester reagent. It is commonplace for the scientist to utilize a Lewis base, such as triethylamine to catalyze this reaction in organic solvent. One of the primary benefits of NHS esters and Sulfo-NHS esters is that they tend to yield reaction products that are specific for the reaction with primary amine residues, such as those found on the N-terminus and on lysine residues in proteins. While other reactions, such as those with hydroxyl groups (-OH) and sulfhydryl groups (-SH) can occur, they tend to occur at a significantly slower rate, and yield reaction products that are easily hydrolyzed or otherwise exchanged for the more stable amide linkage. The reaction selectivity of NHS and Sulfo-NHS esters tends to be for primary amines (-NH2), and this is maximized under pH 7-9 aqueous reaction conditions. It is important to note that any buffers used in these reactions should not contain an interfering nucleophile, such as a Tris or Tris-HCl buffering system. In fact, the addition of Tris-HCl (pH 7.5) or Glycine is an excellent way to quench the reaction, after the reaction has run its course, deactivating the remaining active ester. A common buffering system for these reactions is 100 mM Sodium phosphate with 150 mM Sodium Chloride pH 7.2, which comes pre-formulated and aliquoted in PBS DryBlend pouches (CovaChem 19213). As shown by Sélo et al, the NHS and Sulfo-NHS ester is also capable of being used to more specifically target the N-terminus, rather than Lysine (K) amino groups. This is accomplished by taking advantage of the pKa differences of the two amino (-NH2) groups. The pKa of the ε–amino group of Lysine (K) is about 10.5, while that of the N-terminus is roughly 8.9. By lowering the buffer pH to 6.5, one can take advantage of this difference in protonation state. At pH 6.5, the Lysine ε–amino group would be more fully protonated (-NH3+) and non-nucleophilic, while the protein or peptides N-terminus would still be sufficiently available in the nucleophilic, non-protonated (-NH2) form2. CovaChem manufactures a wide range of protein crosslinkers and protein modification reagents that contain this useful NHS or Sulfo-NHS ester reactive group. Many of our crosslinkers have an additional reactive group, making these molecules heterobifunctional, allowing for the targeting of an additional protein functional group. This gives the user even more control over their protein investigational studies, or bioconjugate formation. Our technical representatives are readily available to assist in finding the right crosslinker or modifier and to aide in developing application specific procedures. References |