Beta Glucuronidase (β-D-Glucuronidase) is an enzyme regularly used for in vitro drug metabolism studies, as well as in routine drug testing applications. β-Glucuronidase is an enzyme classified as a hydrolase, as it is actively involved in the hydrolytic cleavage of drug-glucuronide conjugates, also known as cleaving glucuronide tails. Glucuronide conjugate formation is a common metabolic process utilized by numerous organisms, and is called glucuronidation. Glucuronidation plays many important roles in the human body, including often being a critical component of drug metabolism. Glucuronidation is an enzymatic process that takes place primarily in the liver, and is involved in the removal of toxins, drugs or other xenobiotics from the body. Glucuronidation is the enzyme facilitated process of transferring a glucuronic acid from UDP-glucuronic acid to the xenobiotic substance (Figure 1). Often this results in increased water solubility of the drug or other foreign molecule, allowing for the more efficient excretion from the organism. 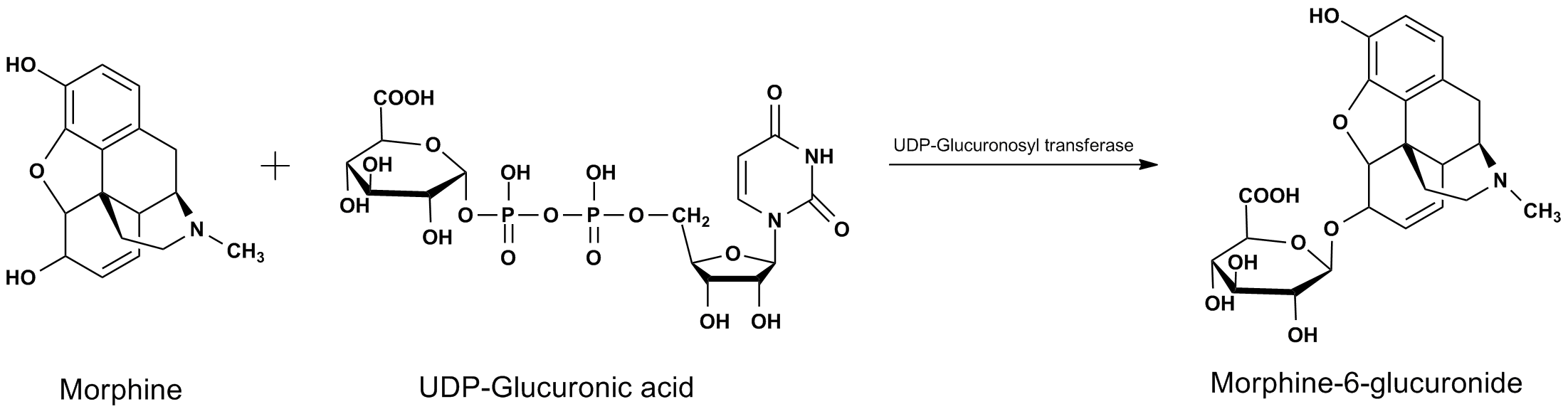 Figure 1. General mechanism for glucuronidation of morphine from UDP-Glucuronic acid. For this reason, when a patient ingests a controlled substance, either legitimately or illegally, glucuronide conjugates, such as Morphine-6-glucuronide will be present in the subject’s blood as well as urine. This fact makes it possible for drug testing laboratories to perform routine screening of urine or blood for the presence of these controlled substances. The glucuronide tag complicates the analysis, and makes direct analysis and quantification difficult. Therefore, it is usually preferable to cleave the conjugate, resulting in the release of the drug compound or metabolite. There are multiple ways in which these conjugates can be cleaved, primarily consisting of chemical or enzymatic processes. The enzymatic approach is typically preferred for complex biological samples or in specific cases where the chemical approach is known to cause the formation of multiple by-products for a given conjugate. Cleavage of the glucuronide-drug conjugate with the β–Glucuronidase enzyme renders the intact de-glucuronated organic compound that has better solubility properties and can more effectively be extracted from the sample. This glucuronide removed compounds can be readily detected by definitive analytical methods, such as liquid chromatography with a mass spectrometer detector (LCMS). Figure 2 illustrates the cleavage of the 6-Morphine-6-glucuronide (M6G) conjugate. After de-conjugation, and prior to analysis, it is often routine for the sample to be further processed through ion exchange chromatography or extraction before the final LCMS analysis is performed. 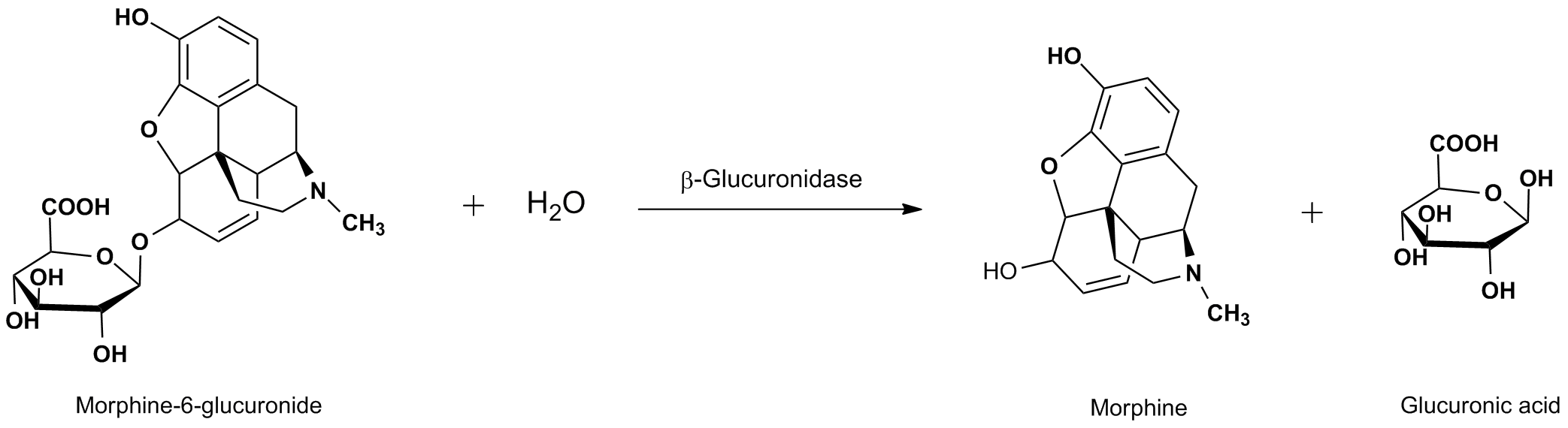 Figure 2. Beta Glucuronidase mediated hydrolysis of Morphine-6-glucuronide. It is important to note, that not all glucuronide-drug conjugates cleave at exactly the same rate, and some conjugates can hydrolyze at rates considerably different than other seemingly similar compounds. For example, it is known that Morphine forms multiple metabolic glucuronides (M3G and M6G). The M6G conjugate has an enzymatic hydrolysis rate similar to that of codeine, and roughly 1/4th that of the M3G glucuronide conjugate.1 Therefore, when developing a method for the cleavage of a wide range of substrates, it is worthwhile to explore conditions that will adequately account for these variations in conjugate hydrolysis rates. For this reason, protocols are often slightly overdeveloped, insuring that a single set of reaction conditions will fully cleave a wide range glucuronide-drug conjugates. Furthermore, depending on the application, it may also be beneficial to experiment with slight changes in temperature (up to about 65 C) when exploring optimal conditions. β-Glucuronidase is obtained through its isolation from its natural biological source. There are many organisms that are rich in Beta-Glucuronidase, and several of these can be used to extract this enzyme. Some examples include: Bovine Liver, E. coli, Helix pomatia, limpets, Escherichia, and Abalone. While there have been a handful of studies comparing β–Glucuronidase sources, it is clear that Abalone tends to give some of the most favorable conversion results, and the least amount of chromatographic interference.2 Abalone as a β–Glucuronidase source is also one of the most economical options, which can be of particular concern in high throughput applications, such as routine drug screening.3 CovaChem produces some of the industries most active Beta-Glucuronidase. This product is available as both a 100,000 U/mL liquid (CovaChem ) and as a lyophilized powder. For product information, see the links below: Beta-Glucuronidase Liquid (CovaChem 20101) For more general information regarding this enzyme, see our Beta-Glucuronidase page. References 1. Comparison of the hydrolysis rates of morphine-3-glucuronide and morphine-6-glucuronide with acid and beta-glucuronidase. Romberg RW, Lee, L. J. Anal Toxicol. (1995) 19(3):157-162. |
Beta-Glucuronidase Solution
Views: 1
Beta-Glucuronidase solution (β-Glucuronidase) is used in drug testing applications. With our low price of 25 mL for ONLY &36;275, CovaChem is re...